“…Anvisa is up-to-date by requesting in vitro toxicological evaluation studies to replace in vivo studies, since in vitro tests have several advantages…”




Tauane de Lima Astolfo is the director of in vitro toxicological studies at SCI-AGRO Laboratory and a biologist from the Federal University of São Carlos.
Ana Carolina Gomes da Silva is the director of in vivo toxicological studies at SCI-AGRO, graduated in biomedicine at Fundação Hermínio Ometto.
Lahys G. Caetano Tomaz is the technical commercial manager at SCI-AGRO. Tomaz holds a degree and a master’s in chemistry from Universidade Estadual Paulista.
Paulo Marcos da Silva is the CEO of SCI-AGRO. Silva is a chemist from the Federal University of São Carlos and master in agronomy from University of São Paulo.
SCI-AGRO is a Brazilian laboratory located in Charqueada, State of São Paulo, recognized in good laboratory practices by Inmetro – OECD and focused on studies for the registration of pesticides.
In Brazil, the registration of agrochemical requires the presentation of a dossier containing, in addition to other documents, reports of toxicological, ecotoxicological and physical-chemical studies. These studies must be conducted under the principles of good laboratory practice (GLP). The dossier is evaluated by three regulatory agencies, MAPA (Ministry of Agriculture, Livestock and Supply), Ibama (Brazilian Institute of Environment and Renewable Natural Resources) and Anvisa (Brazilian Health Regulatory Agency). Anvisa is responsible for analyzing the potential for danger to human health. It is the agency that evaluates toxicological studies.
Toxicological studies currently required for registration of agrochemicals by equivalence are: Gene Mutation in Bacterial Cells, Acute Oral Toxicity, Acute Dermal Toxicity, Acute Inhalation Toxicity, in vitro Chromosomal Damage in Mammalian Cells, Skin Sensitization, Skin Irritation & Corrosion and Irritation & Eye Corrosion.
Considering that in vivo toxicology uses a large number of laboratory animals, the implementation of alternative methods (in vitro) is a global trend, which is based on the principle of the 3R’s, “reduction, refinement and replacement” in the use of animals, to reduce animal suffering. In view of this fact, the National Council for the Control of Animal Experimentation – Concea was created in 2009 (Law n. 11.794/2008; Decree n. 6.899/2009), responsible for monitoring and evaluating the introduction of alternative methods internationally validated (OECD, EPA, etc). Anvisa resolutions No. 35/2015 and Concea No. 31/2016 define alternative methods for carrying out toxicological studies in Brazil.
In 2019, Anvisa resolution RDC 294, which regulates the use of in vitro tests in Brazil, states that in vivo tests can only be performed if the in vitro methods are inconclusive, without classification prediction or if there is a technical-scientific justification that limits the execution of the in vitro study. In the same resolution, Anvisa adopted the “Globally Harmonized System of Classification and Labeling of Chemicals” (GHS) for the toxicological classification of substances based on the results of toxicological studies.
According to Concea, Chromosomal Damage studies in mammalian cells, Skin Irritation/Corrosion, Eye Irritation/Corrosion and Cutaneous Sensitization should initially be conducted in vitro, and depending on the results found, they can be complemented with in vivo studies.
Considering the OECD (Organization for Economic Cooperation and Development) as one of the internationally recognized agencies, its methods can be officially used in Brazil and are exemplified in the table, summarizing the changes in methods after the publication of RDC 294/2019 by Anvisa.
| Studies | Methods Prior to
RDC 294 (2019) |
Methods After
RDC 294 (2019) |
| Gene Mutation in Bacterial Cells | OECD 471- in vitro | OECD 471 – in vitro |
| Acute Oral Toxicity | OECD 423 – in vivo | OECD 423 – in vivo |
| Acute Dermal Toxicity | OECD 402 – in vivo | OECD 402 – in vivo |
| Acute Inhalation Toxicity | OECD 403 – in vivo | OECD 403 – in vivo |
| Chromosomal Damage in Mammalian Cells | OECD 474 – in vivo | OECD 487,
OECD 473 – in vitro |
| Skin Sensitization | OECD 406, 429 – in vivo | OECD 429, 442A, 442B – in vivo
OECD 442C – in chemico OECD 442 D, 442E2 – in vitro |
| Skin Irritation | OECD 404 – in vivo | OECD 439 – in vitro |
| Skin Corrosion | OECD 431 – in vitro | |
| Eye Irritation | OECD 405 – in vivo | OECD 437, 491, 438, 460, 492, 492B2 – in vitro |
| Eye Corrosion | ||
| Gene Mutation in Mammalian Cells 1 | OECD 490, 476 – in vivo | OECD 490, 476 – in vitro |
For the studies of Gene Mutation in Bacterial Cells, Acute Oral Toxicity, Acute Dermal Toxicity, Acute Inhalation Toxicity and Gene Mutation in Mammalian Cells, there was no change prior to the publication of RDC 294/2019 and the methods remained the same after its publication. For the other studies, the guidance described below applies.
Guidance for the use of in vitro x in vivo methods:
The evaluation of Chromosomal Damage in Mammalian Cells must be initiated by the in vitro method OECD 487 or 473. If the result obtained is inconclusive or positive, the study must be complemented by the in vivo method OECD 474.
Gene mutation studies in mammalian cells using the thymidine kinase gene (OECD 490) or the hprt and xprt genes (OECD 476) will only be conducted when required by Anvisa. These studies will not be performed when gene mutation in bacterial cells and chromosomal damage are detected in OECD 471 and OECD 487 studies, respectively.
Skin Corrosion/Irritation and Eye Irritation/Corrosion studies must follow the order of test conduct as guided by the Integrated Testing and Assessment Approach (IATA). Skin Irritation & Corrosion studies must follow the IATA 203 document, according to the flowchart.
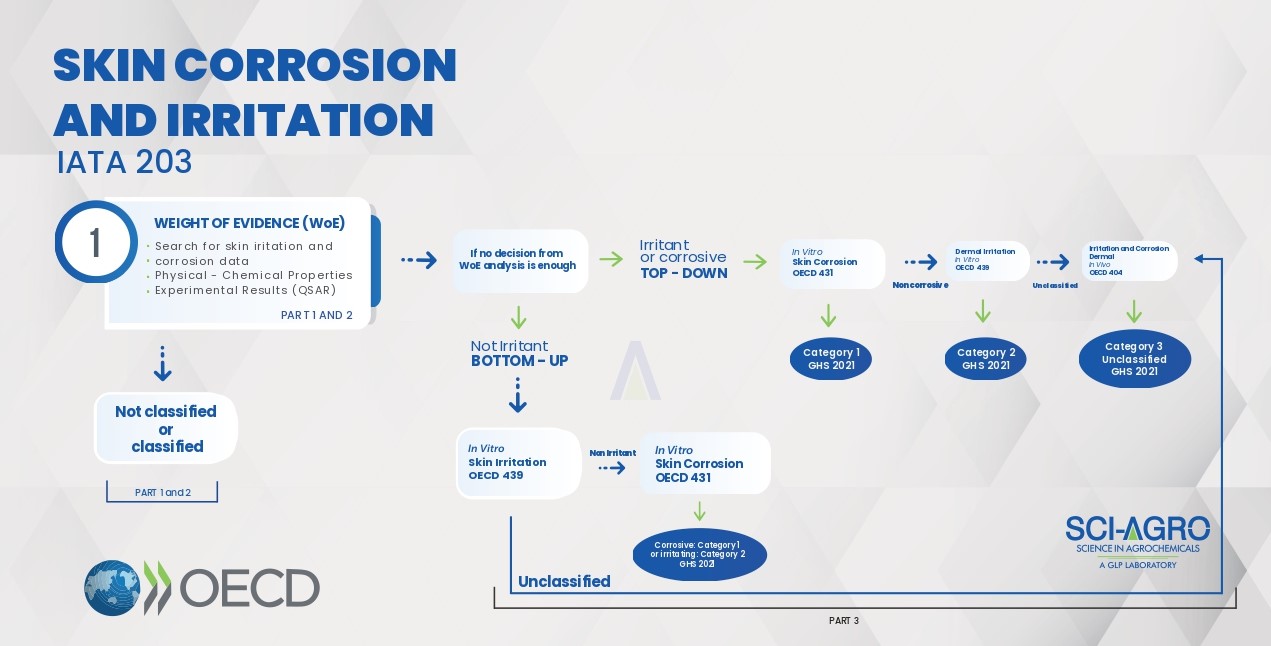
This document guides to initiate the study through the survey of data available (Part 1), to which an analysis of ‘Weight of Evidence’ – WoE is attributed (Part 2). Predictions obtained from the ‘Structure and Activity Relationship’ (Q) SAR may be effective in this preliminary phase of IATA.
In case the data found in Parts 1 and 2 are not conclusive or incomplete, Part 3 (experimental) should be started. It is worth mentioning that the data found in the WoE stage have greater applicability for high purity products, such as technical grade products, since different compositions of formulated products, even if small, can lead to different results from what is found in the literature.
The WoE step can direct the possible classification expected for the product to be tested (Irritant/Corrosive or Not Irritant), guiding the best experimental approach (top-down or bottom-up). At this stage, the use of animals should only be considered as a last resort, being replaced by alternative methods, according to the picture above. For example, for the top-down approach, the study must start in vitro (OECD 431) and end with the in vivo study OECD 404, if inconclusive in the previous steps. Likewise, the bottom-up approach begins with the in vitro study OECD 439 and ends with the in vivo OECD 404.
Eye Irritation & Corrosion studies must follow the IATA 263 document, according to the flowchart.
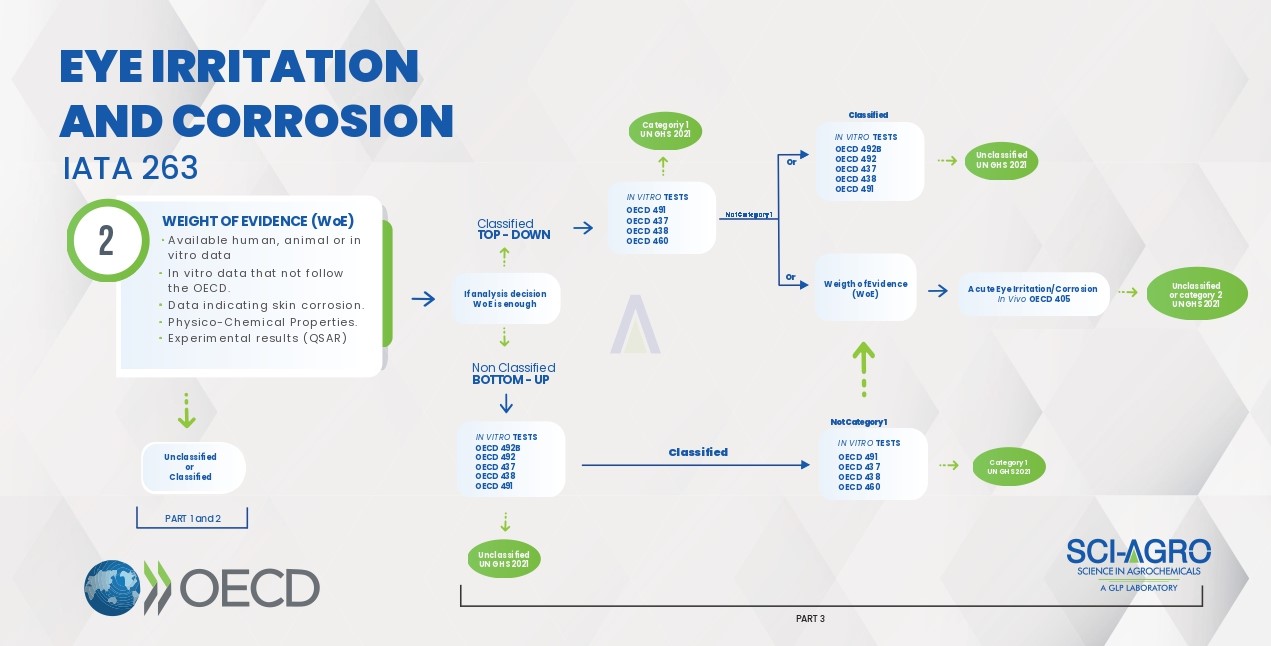
In the same way as IATA 203, this document (IATA 263) also starts with the survey of relevant data available in the literature (Part 1) and analysis of ‘Weight of Evidence’ – WoE (Part 2). In case the data found in Parts 1 and 2 are not conclusive or incomplete, the experimental part (Part 3) must be started according to the classification expected for the product to be tested (Classified or Not Classified), guiding the best experimental approach, top-down or bottom-up, respectively. At this stage, the use of animals should only be considered as a last resort, being replaced by alternative methods according to the figure above. For example, for the top-down approach, the study must be initiated by one of the in vitro methods OECD 491, 437, 438 or 460 and ended with the in vivo study OECD 405, if inconclusive in the previous steps. Likewise, the bottom-up approach starts with one of the in vitro methods OECD 492B, 492, 437, 438 or 491, ending with the in vivo method OECD 405.
For the assessment of skin sensitization, the in vivo methods OECD 429, 442A and 442B can be used in Brazil since they are approved by Concea as alternative methods due to the reduction in the use of animals. For this evaluation, IATA 256 addresses decision-making to facilitate the development of a strategy during the evaluation of the product under test.
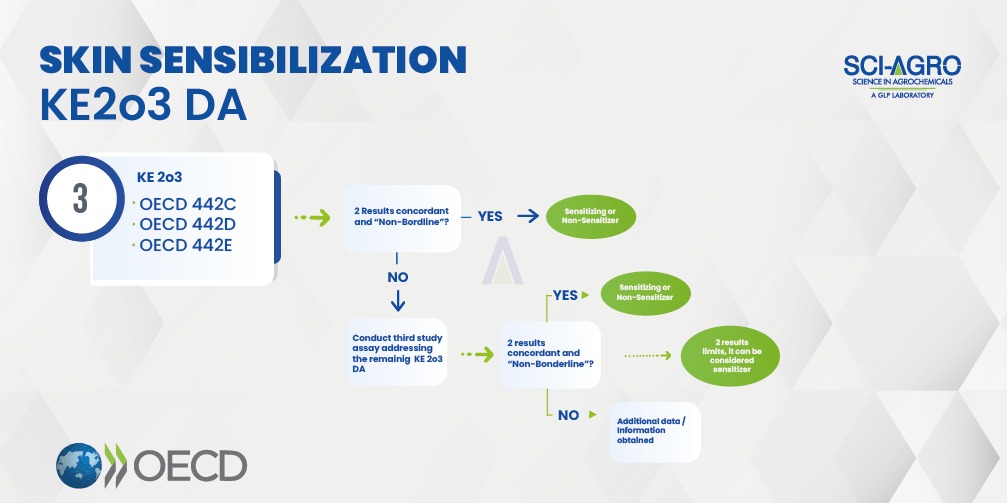
Recently, OECD 497 (June/2021) was published, which is supported by OECD 336 (August/2021). According to this guideline, the sensitizing or non-sensitizing classification can be done through an integrated approach of collecting available data and complemented with experimental tests. OECD 497 describes defined approaches (DA) based on the integration of key events (KE) involved in sensitization mechanisms: a) covalent binding to skin proteins (OECD 442C); b) activation of keratinocytes (OECD 442D); c) activation of dendritic cells (OECD 442E) and d) activation and proliferation of T lymphocytes (OECD 429, 442A and 442B). This approach can identify hazard, which is the discrimination between sensitizers and non-sensitizers, or potency subcategorization (Category 1A = strong sensitisers; Category 1B = other sensitisers and Not Classified).
Skin sensitization studies must be initiated using two out of the three defined methods (KE 2o3 DA), according to the flowchart below, based on OECD 497. In vivo methods (OECD 429, 442A and 442B) have an accuracy of approximately 72%, but the KE 2o3 DA approach (OECD 497) achieves a precision of over 72%, as the combination of tests increases data. It is also important to combine the tests with other sources of information, such as Weight of Evidence – WoE.
Anvisa is up-to-date by requesting in vitro toxicological evaluation studies to replace in vivo studies, since in vitro tests have several advantages, mainly in terms of reducing the number of animals. However, in vivo studies have their importance and credible results and, due to this, they are used when there is a need to confirm in vitro studies. Anvisa may request, whenever it deems necessary, complementary in vivo studies. More alternative methodologies will be validated and will be accepted by regulatory agencies.
References
Anvisa. Constituição (2019). Resolução da Diretoria Colegiada nº 294, de 29 de julho de 2019. RDC. 146. ed. Seção 6, p. 78-85.
Anvisa. RESOLUÇÃO n. 35, de 07 de agosto de 2015. Diário Oficial da União, 10 de agosto de 2015.
BRASIL. Concea. RESOLUÇÃO n. 31, de data inválida. Diário Judicial Eletrônico. PORTO ALEGRE, 28 de abril de 2016.
UN (2021). United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (ST/SG/AC.10/30/Rev.9). United Nations New York and Geneva, 2021.

