Required data, costs and timeline, by Patrícia Karlikowski.
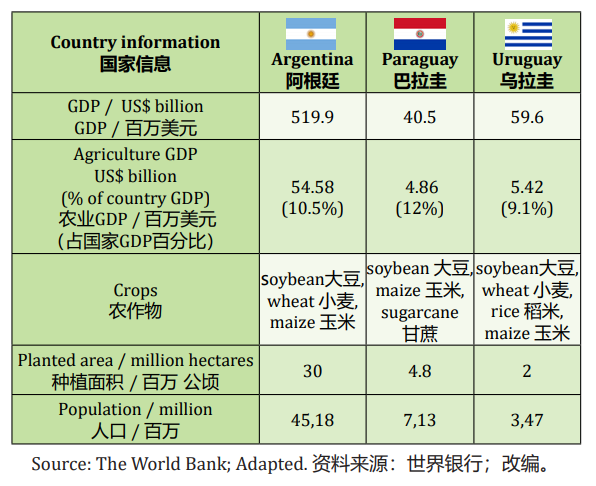
Soybean is the main agriculture commodity produced and exported in the extreme South region of Latin America. Argentina, Brazil, Paraguay and Uruguay, with highlight for the 3 first, together are responsible for
almost 55% of the harvested soybean in the world, in an approximated area of 56.8 million hectares. The use of modern technology and tools to improve yields include GMO seeds, fertilizer, pesticides. Patrícia Karlikowski, in this article to AgriBrasilis, explains pesticide registration characteristics and issues in Argentina, Paraguay and Uruguay.
Patrícia is graduated in International Business from Argentine University of Business (UADE), has a postgraduate degree in Agrifood Foreign Trade from University of Buenos Aires (Agronomy Faculty –
FAUBA) and specialization in Agribusiness from Universidad de Buenos Aires. Patrícia is currently
Corporate Head of Projects and Regulatory Affairs in DVA Agro GmbH, in Buenos Aires (Argentina).
ARGENTINA
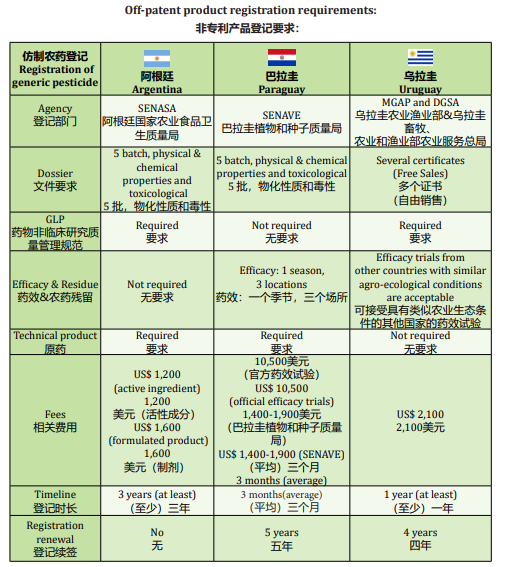
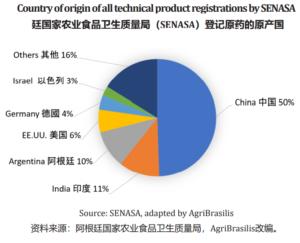
SENASA (Servicio Nacional de Sanidad y Calidad Agroalimentaria) is the only and main regulatory authority responsible for executing national policies on animal and plant health and quality and food safety within its competence, as well as verifying compliance with current regulations on the matter. Resolution No. 350/99 is the main one that regulates registration process. There are also many additional resolutions that complements the 350/99. Registration of crop protection products refers mainly to technical product registration on one hand, and formulated product registration on the other hand. There are many different stages and important parts to success on the registration approval. New sources or new crops in the label implies different requirements and processes that companies must fulfill.
Something common in all types of registrations is that we, all companies and its representatives, have to demonstrate that the sources are real manufacturers; this means that exists the manufacturing site with licenses and certificates that proves the production capacity of these products. This is a key point when we start any registration process.
Technical product registration involves the submission of a dossier with the 5 batch, physical & chemical properties and toxicological reports. All must be performed under GLP accredited laboratory. In addition to other confidential documentation that supplier must share with the registrant. Registration of formulated crop protection products require complete GLP data of Physical & chemical properties, physical and chemical properties related to the use as well as toxicological and ecotoxicological studies. For this type of products, it has to make a differentiation:
1 – products that already exist in the market, called “equivalence process” and,
2 – products that are innovated in the market.
This is important to mention, in the second case, we have to think in a higher investment, since more requirements should be accomplished as well as more time to develop the product and launch the product to the market. New development or innovation products, strongly compromises the agronomic expertise of the registrant. SENASA requires two seasons of efficacy trials, and each of them requires three different agro-ecological locations.
When should we think about residue? When our product exceeds the residue limit already registered and published by SENASA. Adjuvant registration in Argentina has a similar process as crop protection product. Only some few differences must be attended to at the moment of preparing the dossier. Registrations do not require any renewal process except for some annual or biannual fees. It is a cost request, not a documentation process renewal request. Main fees are around US$ 1,200 for active ingredient and US$ 1,600 for formulated product.
For registration approval, it can considered an average of 3 years. Not less. Every process has its own complexity and this is where we have to consider a challenge to accomplish: increasing the human resources, number of companies have increased as well as registrants, and therefore the time to get a dossier evaluated is behind and evaluators are also overworked. Good points to mention, SENASA started approximately two years ago to implement online remote procedures (TAD) for different administrative submissions. It was the first online system in the South Cone region. Although it has made possible to streamline certain processes, we believe that still needs to be improved, including other important areas of
evaluation.
SENASA is working on a new traceability system that is about to come out, demos were already tested and only finalizing details of the databases for crosslinking. This system will be linked to the containers/ packaging data until their final disposition.
PARAGUAY
In Paraguay, we have SENAVE (Servicio Nacional de Calidad y Sanidad Vegetal y de Semillas). SENAVE will be the body for the application of international conventions and agreements related to plant quality and health, seeds and the protection. Registration of crop protection product in Paraguay unlike Argentina,
refers to one submission containing information on both, technical product and the formulated product.
Even though it is one dossier, registrants must submit both technical and formulated products data.
GLP data is not required, nevertheless studies must be carried out according to Internationally Recognized
Methods by Laboratories registered with SENAVE. The dossier of the technical product should include 5
batch, physical & chemical properties and toxicological data. Besides other confidential documentation, that supplier must share with the registrant. About the formulation, the dossier should include data of physical & chemical properties, physical chemical properties related to the use as well as toxicological and
eco-toxicological studies.
Here it is also important to differentiate products, which already exists in the market from those, which are new products. New products require efficacy trials (1 season, 3 locations) and it must be developed by an official entity. Registrations are permanent and fees are around US$ 10,500 for official efficacy trials and USD 1,400 – 1,900 for SENAVE fee. The evaluation time we can consider an average of 2-3 months. The pandemic global situation has raised one of the main problems, which I could say has also moved to other countries, delaying or even stopping all the management.
SENAVE is working in digital management for the registration for all kinds of products (fertilizers, agrochemicals and coadjuvants). Probably new electronic processes will be implemented soon.
URUGUAY
MGAP (Ministerio de Ganaderia Agricultura y Pesca) and DGSA (Dirección General de Servicios Agrícolas) are the competent authorities for the regulation, analysis and control of plant protection products that are being commercialized in Uruguayan territory Only formulated crop protection product is registered. The information and characteristics of the active ingredient (physical chemistry, and a detail of 5 manufacturing batches), are included in the application for registration the formulated product.
Initially, a declaration must be made according to the forms and requirements contained in the Resolution No. 317/007 of the MGAP and its instructions from the DGSA. This declaration contains technical information of the formulated product as well as the active ingredient. This declaration must be accompanied, through annexes, with the physical, chemical reports as well those related to the use. Many
certificates, among them, Free Sales certificates, should be prepared by the origin of the registration (manufacturer), which should also be legalized by legal authorities.
Once this first step is approved, a sample of the product must be entered into the DGSA, along with its respective analytical standard. This is also applicable in Argentina both for technical and formulated
products registrations. This stage is important since all samples are being analyzed and if any does not comply with what was initially declared, will be rejected and another should be submitted in some specific period of time.
Registration is valid for 4 years. Registrant must keep in mind these dates since if a renewal is not being done on time and form, the registration will be automatically discarded, lost, and a new registration process should start again. The process requires GLP data/studies, physical & chemical as well as
data/studies related to the use, toxicological and ecotoxicological.
Different as in Argentina or Paraguay, in Uruguay are accepted efficacy trials from other countries with similar agro ecological conditions. If no trials are available, it can be done in Uruguay. If we talk about fees, they are around US$ 2,100 between the two main regulatory authorities: DGSA and CIAT. We can consider an average of 1 year to get the approval, not less, after submitting the complete information. In this country it is also a big challenge to proceed with electronic ways and counting with more human resources to accomplish a more agricultural demand.